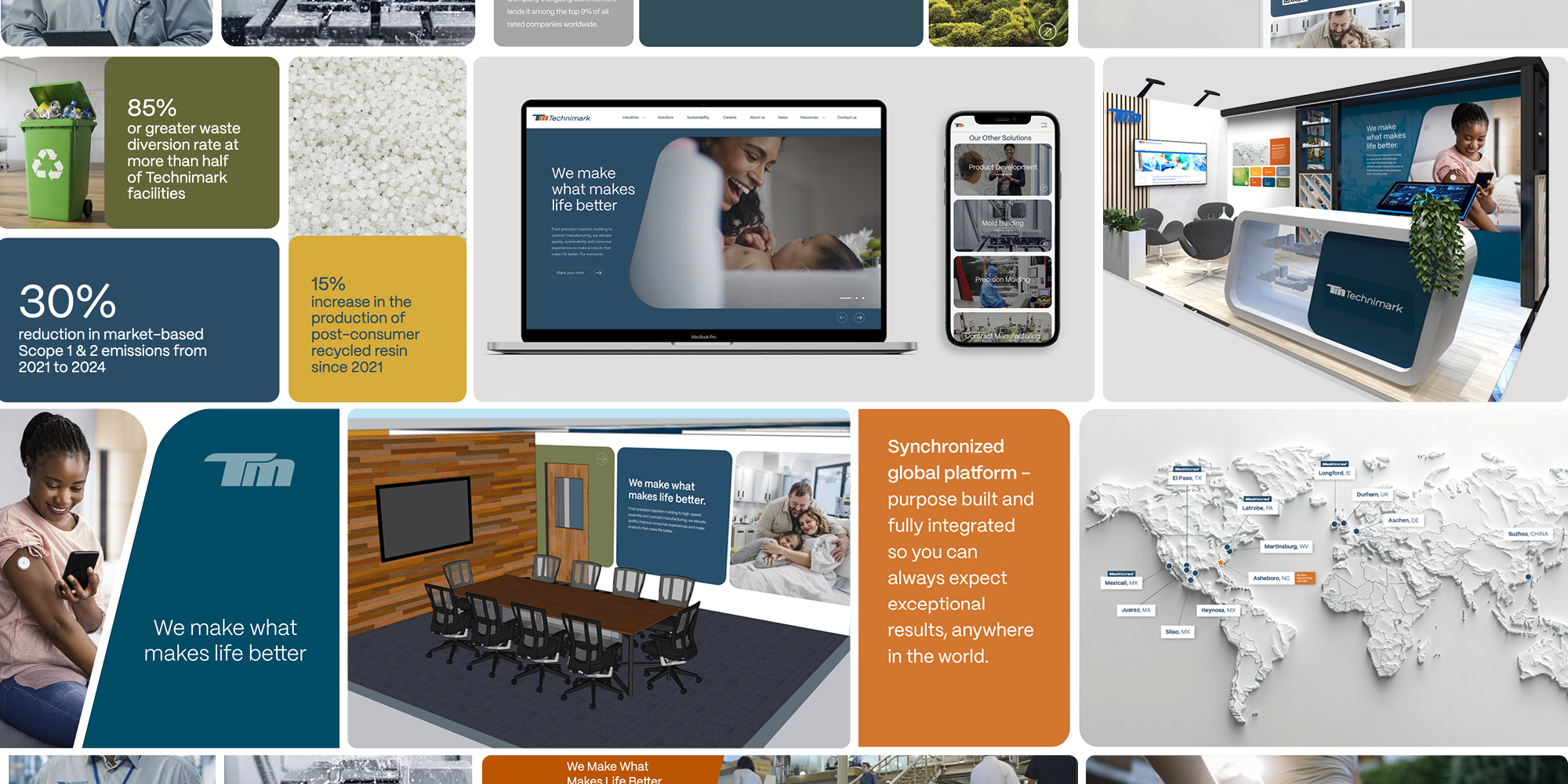
Technimark Unveils New Brand Identity: “We Make What Makes Life Better”
Technimark has introduced a new brand identity that underscores its evolution as a global manufacturing…
Tag
press release
Technimark has introduced a new brand identity that underscores its evolution as a global manufacturing…

Technimark announces a commitment to the Science Based Targets initiative (SBTi) to reduce greenhouse gas…

Mexicali marks the fourth plant that is MedAccred certified demonstrating the strict standards of Technimark's…

PRESS RELEASE: Technimark announced today the release of its 2023 Sustainability Brief, unveiling ambitious 2030…

PRESS RELEASE - Technimark announced today that its production facility in El Paso, Texas, received…

PRESS RELEASE - NEW YORK, June 7, 2021 - Oak Hill Capital, a New York-based…

Technimark is pleased to announce that Kris Peavy, Technimark’s Chief Commercial Officer, has also been…

With its recent acquisition of Ci Medical Technologies, Technimark, a global manufacturer of custom rigid…

Technimark would like to announce the promotion of John Rugari to Vice President of Sales,…