
Technimark Leading the Way in Sustainable Manufacturing
Asheboro, NC – JULY 23, 2025 – Technimark, a global leader in plastic injection molding…
Tag
news
Asheboro, NC – JULY 23, 2025 – Technimark, a global leader in plastic injection molding…
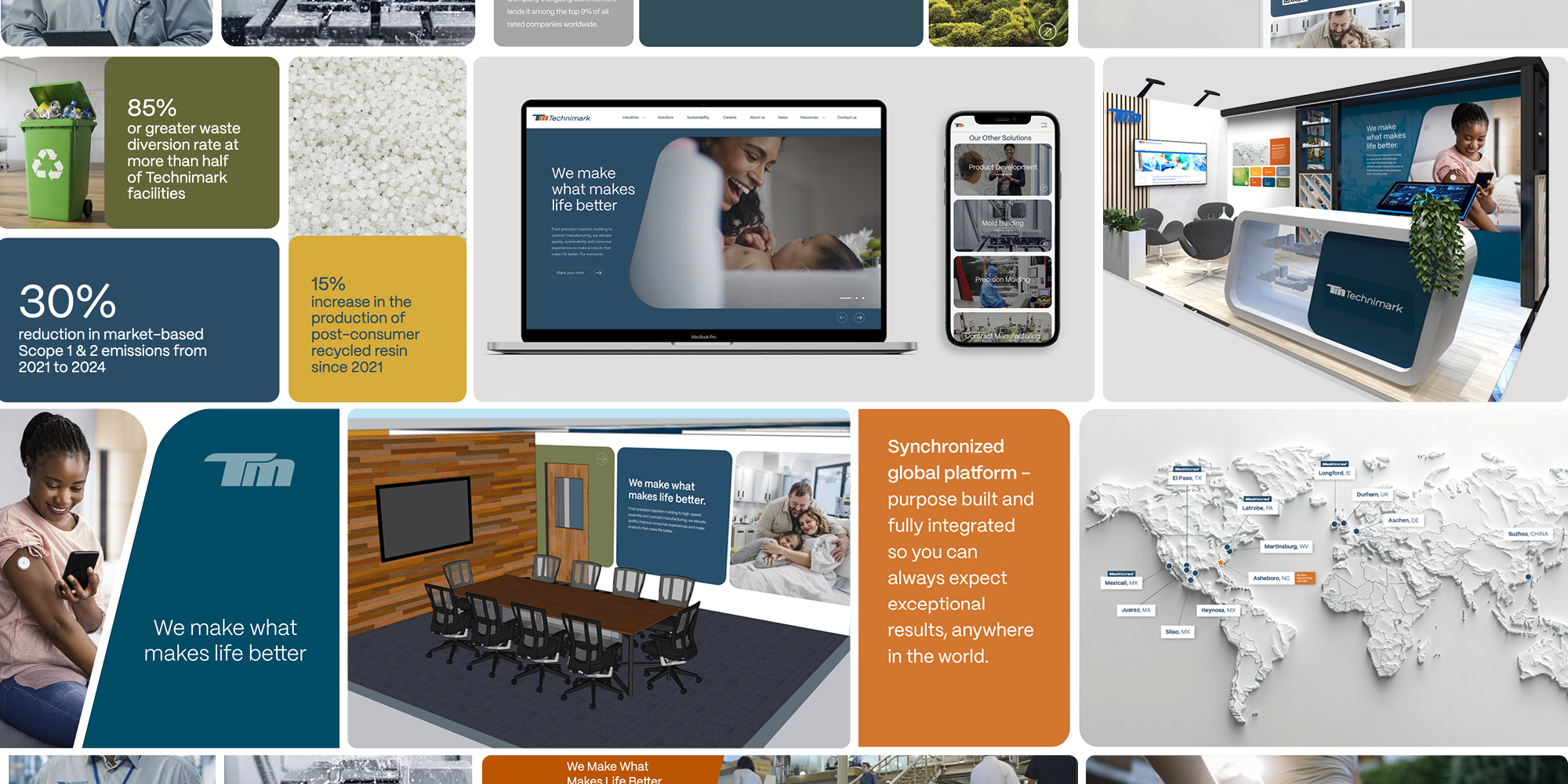
Technimark has introduced a new brand identity that underscores its evolution as a global manufacturing…

Proud to celebrate the talent driving our future. We’re thrilled to announce that Cody Fogleman, Quality Engineering…

Mexicali marks the fourth plant that is MedAccred certified demonstrating the strict standards of Technimark's…

PRESS RELEASE: Technimark announced today the release of its 2023 Sustainability Brief, unveiling ambitious 2030…

PRESS RELEASE - Technimark announced today that its production facility in El Paso, Texas, received…

PRESS RELEASE - NEW YORK, June 7, 2021 - Oak Hill Capital, a New York-based…

PRESS RELEASE - Technimark has partnered with Rhinostics and a P&G subsidiary, iMFLUX, to produce,…

Technimark is pleased to announce that Kris Peavy, Technimark’s Chief Commercial Officer, has also been…